Debt Case Study: Immunic AG
EUR 25m European Investment Bank Infectious Diseases Finance Facility

Company Description
- Immunic AG (Immunic) is a clinical-stage biopharmaceutical company with a pipeline of selective oral immunology therapies aimed at treating chronic inflammatory and autoimmune diseases
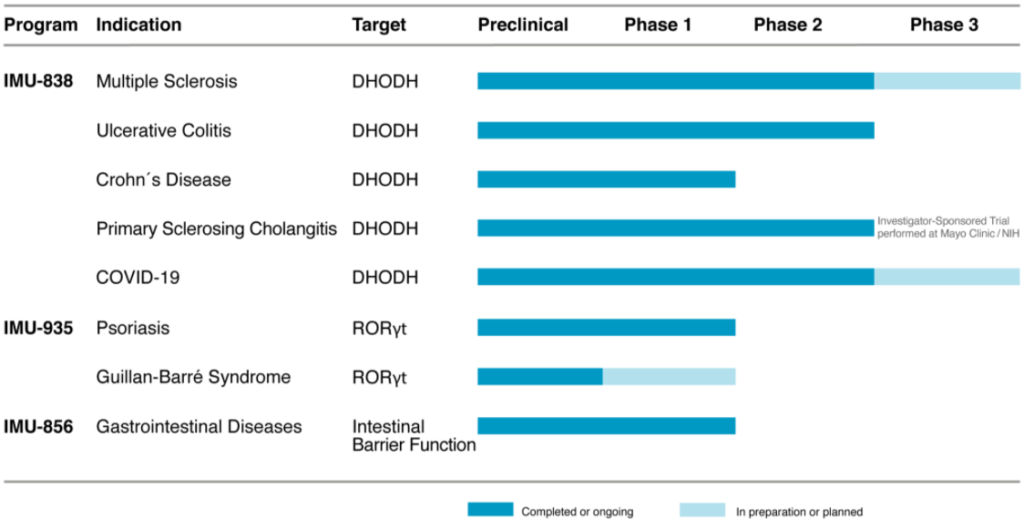
- The company develops three small molecule products including relapsing-remitting multiple sclerosis, ulcerative colitis, Crohn’s disease, and psoriasis
- Its lead development program is IMU-838, a selective immune modulator that inhibits the intracellular metabolism of activated immune cells by blocking the enzyme dihydroorotate dehydrogenase, which is in phase 2 clinical development for treatment of ulcerative colitis,
relapsing-remitting multiple sclerosis and Crohn’s disease - Immunic is also evaluating IMU-838 as treatment for COVID-19
- Founded in 2016 and headquartered in Munich, Immunic currently has approximately 26 employees and has been listed on NASDAQ since 2019
Transaction Highlights
- In order to finance the R&D expenses of its COVID-19 program in the
amount of EUR 50m, Immunic was required to raise additional funds
either from public capital markets or through growth debt - FCF introduced Immunic to the Infectious Diseases Finance Facility
(IDFF) prioritizing COVID-19 programs at that time - The EIB granted Immunic a debt facility of ~EUR 25m to finance its
phase 2 study in COVID-19, to further support a phase 3 study as well
as the commercial production of IMU-838 - For Immunic, the most compelling advantages of the debt facility were:
- The EIB as a financing partner with long-term horizon
- The non-dilutive nature of the loan
- Tranches tailored to the business liquidity needs
- Standard market rates are included, i.e. cash, deferred and
performance-based interest rates
Drug Pipeline*
*as of 12/01/2021
FCF’s Role in the Transaction
- Direct access to relevant sector team at the EIB
- Management of accelerated financing process through proactive preparation of required tax, finance, business and regulatory documents
- Planning of an EIB-specific R&D budget
- Development of a purpose-built business case and EIB-specific, integrated financial model
- Support in the commercial, financial and tax due diligence process
- Negotiation of the term sheet and support in the finalization of financing contract